This web page was produced as an assignment for an undergraduate course at Davidson College.
Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity
Summary
In this article, Markle et al. demonstrate that sex hormone and microbes directly interact. Specifically the article focuses on the incidence of type 1 diabetes (T1D) in high-risk mice, and demonstrates that the presence of a distinct microbiome allows for testosterone-dependent protection from T1D. This was demonstrated through a myriad of experiments. The first important experiment performed by the researchers demonstrates that commensal microbes in the male rodent protect it from T1D. Then, investigators altered the microbiota of female mice by transferring the cecal content of other rodents. The study found that when female weanling recipients received microbiota from male and female donors, the microbiome composition of the host was altered. Researchers then further studied the effects of manipulating the microbiome of a female mouse. The results found that female recipients of a male microbiome had higher testosterone and metabolites concentrations than the control and other experimental groups. Lastly, the researchers demonstrated that testosterone from a male microbiome played a vital protective role against autoimmunity. This was experimentally proven with experiments involving insulitis, Aab production, and the ability of T cells to transfer T1D.
Figures
Figure 1
In panel C, the researchers wanted to learn how different microbial conditions influenced testosterone levels. This was accomplished by quantifying the amount of sex hormones in the serum of both sexes of mice in GF and SPF conditions. There was no difference in 17b-estradiol levels between both sexes of mice in the GF and SPF conditions. However, as seen in panel C, testosterone levels between SPF and GF conditions varied significantly for both males in female mice. SPF male mice had markedly greater levels of testosterone than GF males. For females the opposite was true. The significance of this panel was that it demonstrated that the microbiome in the SPF condition controlled testosterone levels in some sort of fashion.
Next investigators determined if mice being housed in SPF or GF conditions lead to differential metabolomes. This was accomplished by assessing the quantity of 183 serum metabolites using mass spectrophotometry for each experimental condition for both sexes. Scientists found that 55.8% of the total variance observed could be attributed to a subset of glycerophospholipid and sphingolipid metabolites. As seen in panel D, a principal components analysis (PCA) was used to observe the clustering of the experimental conditions. PCA is a method that standardized genomic data that could be represented with three or more dimensions and establishes two orthogonal axes that go through the maximum variation in the data. Data points are then plotted along these two axes. A thorough explanation of PCA can be found at Ordination Methods for Ecologists at Oklahoma State University. These results demonstrate that SPF male and SPF females showed marked clustering. But males and females in GF conditions did not show distinct clustering. The significance of these results was that differences in the microbiome due to sex-specific characteristics influenced a mouse’s metabolome.

Figure 2
In Panel A of this figure, researchers identified microbiome composition differences in male and female mice as they age. This was done by sequencing bacterial 16S rRNA of SPF male and female mice during weaning, puberty, and adulthood. This data was then applied to PCA. The results of the PCA plot demonstrated that as both males and females progressed through the developmental stages, the microbiome composition of each sex become more distinct. The progression starts with weaning mice, where the males (turquoise) and females (red) at this developmental stage have indistinguishable microbiota. However at puberty, the males (pink) and females (green) begin to have more distinct microbiota,. Until at adulthood, the males (blue) and females (black) microbiota are fully distinct clusters. The significance of this panel is that it demonstrates that sexual maturation plays a vital role in the composition of the gut microbiome.
Researchers tested to see if the microbiota transplanted into the female weanlings initiated the production of specific antibodies for the exposed bacteria. This was done by using positive control sera and observing, shown in panel B, the antibody response of IgG1 and IgG2b to the control and female recipients of microbiota relative to the primed controls. Researchers found that female recipients of male or female microbiota did not producing antibodies specific for the bacteria. This means that the microbial exposure did not result in protection from T1D, as previous research had otherwise demonstrated.
In these three panels of Figure 2, the researchers wanted to know if transferring the cecal content of other mice would alter the microbiome composition of a weaning SPF female. This was accomplished via the gavage of adult male and female cecal content to a female host and then using 16S rRNA sequencing to analyze the composition. The figure demonstrates fold changes in the amount of specific bacteria, as well as the phylogeny of the bacteria. Panel C compares fold changes in the microbiome of a control male compared to a control female. Panel D compares a female recipient with a male microbiome to an unmanipulated female and panel E compares a female recipient of a male microbiome to a female recipient of a female microbiome. The main point of these three panels is that the transfer of either a male or female adult cecal content altered the microbiome composition of the weaning female host (panels D and E) compared to the female control.
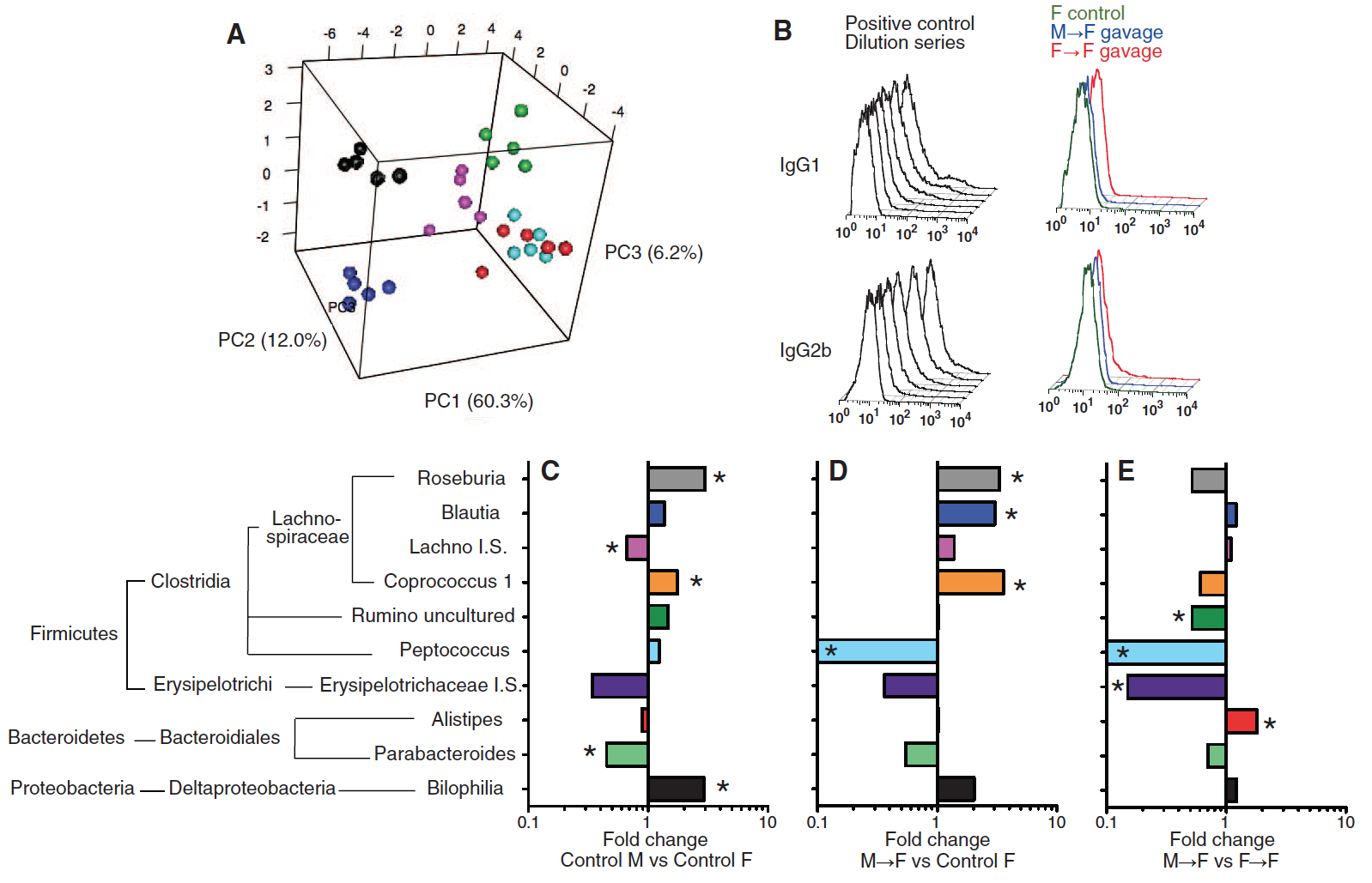
Figure 3
In Panel A of this figure, the scientists wanted to know how transferring a male or female microbiome would affect the testosterone levels of the weaning female host. This was accomplished by analyzing the serum testosterone in recipients of adult male and female cecal content. Scientists found that female recipients of a male microbiome had significantly higher levels of testosterone than the unmanipulated females and same aged female hosts who received a female microbiome. This trend was observed for female hosts that were 7 and 14 weeks old, but not the 34 week old mice.
The investigators considered the results they found in panel 1D that demonstrated that male and female mice in SPF conditions had distinct metabolite compositions. Now, researchers wanted to determine differences in metabolite concentrations in a female recipient of a male or female microbiome. 183 metabolites were quantified in the sera of unmanipulated females, female recipients with a female microbiome, and female recipients with a male microbiome with and without the simultaneous treatment of flutamide. Flutamide is an androgen receptor antagonist, meaning it will prevent testosterone from interacting with its receptors and inhibit the hormone from having an effect. In the PCA plot, it is apparent that the female recipients with a male microbiome (panels C and F) have a distinct metabolome from the unmanipulated females (panels B and E) and the experimental condition treated with flutamide (panels D and F). It is important to note from this figure that female recipients with a male microbiome that were simultaneously treated with flutamide had a similar metabolome to female recipients of a female microbiome. This indicates that flutamide blocked testosterone and prevented it from playing a key role in altering the metabolic activities.
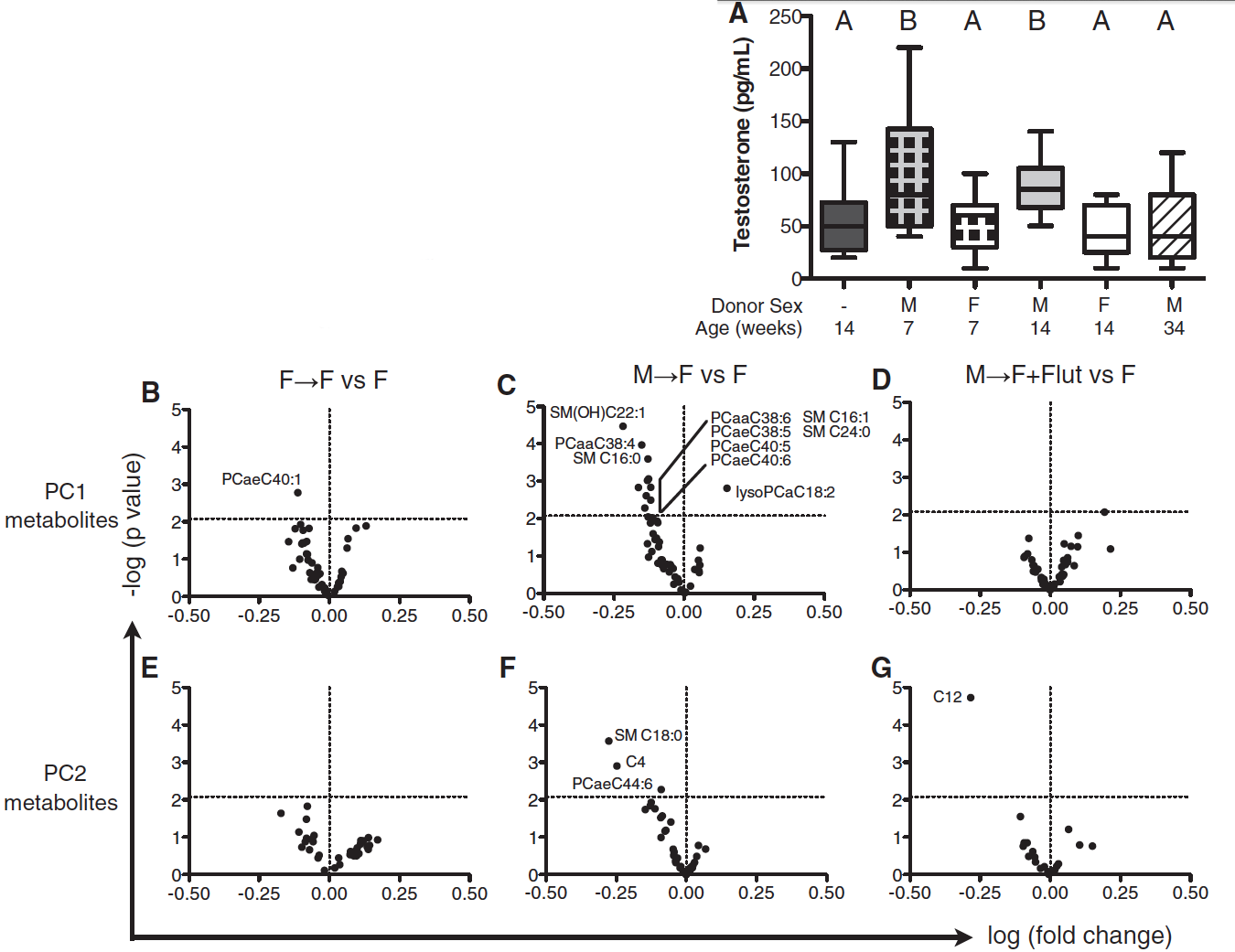
Figure 4
In panel A of this figure the researchers wanted to investigate if the transfer of male microbiota changed the autoimmunity of the recipient. In order to test this, three conditions were created. The control condition included a group of NOD females that were unmanipulated. The two experimental groups were weaning NOD females that were given either male or female microbiota. The results demonstrated that only female hosts that received a male microbiome had a significantly lower incidence of T1D and were therefore protected from the autoimmune disease. The unmanipulated female and female recipient of the female microbiome shared the same incidence of T1D.
Since insulitis progresses before the onset of T1D, researchers wanted to investigate the severity of insulitis in mice with alerted microbiota. In order to assess this, 14-week-old mice from five separate cohorts were examined for the severity of insulitis. The severity of insulitis was scored on a scale of 0-4, where a score of 4 was the most severe. These cohorts were: the unmanipulated females, female recipients of the female microbiome, female recipients of the male microbiome with and without flutamide treatment, and a control cohort of females recipients of sterile male cells only. From panel B, it is demonstrated that treating females with sterile male cells did not deter islet progression, meaning that orally delivering male antigens did not elicit an immune response. It is important to note that female recipients of the male microbiome had significantly lower insulitis severity scores than the unmanipulated females and female recipients of the female microbiome. However, this protection was lost when female recipients of the male microbiome were simultaneously treated with flutamide. The meaning of this result is that testosterone was integral to the protection from insulitis. Since when the androgen receptor was blocked by flutamide, the testosterone received from male donor microbiome was unable to protect the female host.
The purpose of panel C is to further support the findings in Panel B. In this panel, the investigators measured the amount of Aab produced, which is an anti-insulin antibody, in each of the cohorts used in the previous panel. The results demonstrated that only female recipients of a male microbiome had nearly no Aab production, meaning that this cohort did not present the autoimmune phenotype. This production of Aab for this cohort was significantly lower than the Aab production for unmanipulated females and female recipients of female microbiota. Similar to panel B, when the female recipients of male microbiota were treated with flutamide, an Aab antibody production similar to the unmanipulated females was measured. This means that when the cohort was treated with the androgen antagonist, the cohort was not protected and presented the autoimmune phenotype. This is important because it only further emphasizes that testosterone is vital to protecting the female mice from T1D.
In the last panel of this figure, panel D, the researchers wanted to explore if the microbiome manipulations altered the potential of T cells to cause T1D. This was done by intravenously injecting the splenic T cells from unmanipulated females and female recipients of male microbiota with and without the treatment of flutamide to lymphocyte-deficient NOD.SCID recipients. Then the ability of these T cells to transfer T1D was measured. The results found that the lymphocyte-deficient recipients developed T1D at similar rates when injected with T cells from the unmanipulated females and female recipients of a male microbiome that were simultaneously treated with flutamide. But T cells that originated from female recipients of a male microbiome had a delayed ability to pass on T1D. Since the delay in the onset of T1D disappeared whenever flutamide is present, which blocks the activity of testosterone, it is evident that testosterone is vital to the autoimmune response.
Opinion
I thought that this paper was a very intriguing one to read. The paper fluidly creates a link between the microbiome that exists inside a rodents gut, sex hormones, and autoimmunity. I particularly found this paper exciting because of its focus on the microbial population that exists inside the gut of all mammals. My excitement stems from the fact that while microbes are the most abundant cells in our bodies and heavily populate the digestive system, little is still known about the microbiome and its interaction with the body. This study was one of the first studies I have ever read that made a genomic connection between the microbiome and its interaction with the host. I would like to see further research to delve into how testosterone protects against T1D and possibly study the human microbiome interactions with other signaling molecules in our bodies.
This paper did a very thorough job of presenting a convincing case. The main strength of this paper is how the researchers validated a lot of their findings with additional experiments and added components to their experiments to rule out extraneous sources of error. For example, in Figure 4B the investigators show that testosterone was fundamental in reducing the severity of insulitis, which precedes the onset of diabetes. This portion of the figure demonstrates that testosterone plays a protective role against the development of T1D. Yet, the researchers perform another experiment that measures the production of anti-insulin antibody Aab. By performing this experiment and reporting the results, the researchers were able to further validate with greater upmost confidence that the testosterone had protective traits against autoimmunity.
A minor weakness of the paper is that at times it fails to demonstrate data or evidence for a few helpful conclusions. For example, in the beginning of the study they introduced an altered Schaelder flora to the GF NOD mice and claim to observe a sex depend trend on the incidence of T1D. This information was used to reassure that there was some sex bias in T1D and therefore encourage the pursuit of sex-specific characteristics. It would have made the paper more compelling if they were to include this data or the figure rather than have it in the supplemental information. However, the exclusion of such a figure does not weaken the final conclusions of the paper, so its absence is not detrimental. Regardless, the logical flow of ideas and progression of evidence throughout the paper makes this a very strong and compelling paper.References
Email Questions or Comments to gacambronero@davidson.edu
© Copyright 2014 Department of Biology, Davidson College, Davidson, NC 28035